As you can imagine, 2020 saw a huge reduction in speaker dinner programs due to COVID-19. The Office of Inspector General (OIG) and the Department of Justice (DOJ) took the opportunity to let biopharma know that these programs remain risky. In its most recent guidance, it stated, “Companies should assess the need for in-person programs given the risks associated with offering or paying related remuneration and consider less-risky means for conveying information to HCPs.”1 While the purpose of this article is to provide insight on how virtual speaker programs can reduce the risks while continuing to deliver education, let’s first review why speaker programs are conducted in the first place.
Are you old enough to remember when socializing with healthcare professionals (HCPs) was an important aspect of building trust? HCPs have always been more willing to consider new treatment options for their patients when they’ve trusted the source of their information. High-tech innovations require education in order for HCPs to utilize novel biopharma solutions in their practice.
Speaker programs are created to disseminate important information on biopharma technologies which ultimately may lead to better patient care. When participating, an HCP wants to know and trust the source. Twenty-five years ago, we built trust in different social environments than we do today. I can remember walking through the Philadelphia Museum of Art with HCP customers before a speaker program. To me, this was an event that allowed people to get to know each other to help facilitate trust. As a field representative and manager, I was grateful to get to know my customers; and personal relationships go both ways. Customers who knew me were more likely to trust the insights that I could bring to them. That trust would open the doors for me to have conversations with medical science liaisons and/or key opinion leaders (KOLs) about treating patients. In addition, I held myself accountable to truthfulness and integrity because, if I didn’t, my relationships could be lost. In my experience, I do believe that my customers only prescribed a treatment if they felt that it was beneficial to their patients.
Most companies ended this type of socialization and entertainment in the 1990s or early 2000s, however, peer-to-peer education was necessary and continued on.
In 2003, when the OIG of the Department of Health and Human Services continued its efforts to promote voluntary compliance, biopharma companies developed and implemented internal controls and procedures. The industry changed the way it was delivering speaker programs as entertainment had long been gone, and to me, so many of the changes were a breath of fresh air because they curtailed egregious activities. Most recently, in November 2020, the OIG delivered, “Special Fraud Alert: Speaker Programs,”1 prompting regulatory professionals and compliance officers to ask themselves, “how can we find new ways to reduce our risks with speaker programs?” The answer is quite simple: virtual speaker programs.
Brian Dahl, president of Dahl Compliance Consulting, believes that there are clear reasons why this guidance is being delivered now. Mr. Dahl commented, “there have been significant enforcement actions, such as with Novartis in July 2020, which settled with the DOJ for $642 million to resolve allegations that included conducting an inappropriate speakers program.2 The OIG wants to send a clear message that they are expecting that once we get past the COVID-19 pandemic, biopharma companies should not go back to business as usual with speaker dinner programs.”
The chart below represents characteristics that the OIG wants addressed. While there isn’t much new in this guidance that wasn’t already discussed in the 2003 OIG Compliance Program Guidance for Pharmaceutical Manufacturers,3 it’s clear that they want pharma to take a closer look at how this guidance is being followed and if, due to what we’ve seen during the COVID-19 pandemic, programs are in fact seen as necessary.
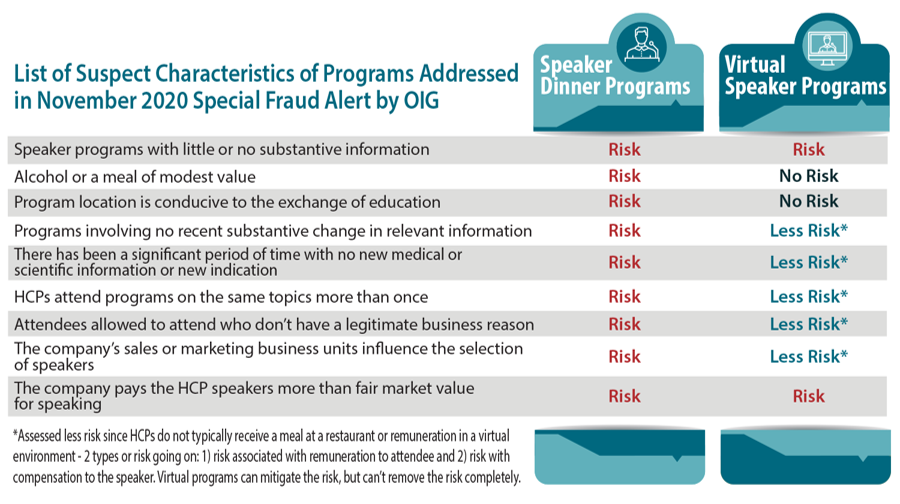
When looking at the chart, speaker dinner programs have the potential of being risky from every suspect characteristic that is listed by the OIG/DOJ. The level of risk a company is willing to take must be determined by each biopharma company. However, if a company is concerned that the risk of conducting speaker dinner programs outweighs the benefits, then virtual speaker programs can deliver the needed education while lessening the number of risks taken.
During COVID-19, our clients moved with great success toward a virtual environment for their programs. Not only are virtual speaker programs less expensive and more customized for the HCP in need of information, they reduce many of the risks that were concerning to the OIG/DOJ. By simply not providing a beverage or meal at a restaurant, they gain only the knowledge shared.
Speaker dinner programs still have an important place in the world of healthcare education. As the OIG suggests, perhaps they are to be used when there are new products, new indications, and new guidance is in place. While we cannot eliminate all risks without ceasing direct in-
person contact with HCPs, it’s important to ask ourselves, “how else can we continue to deliver valuable peer-to-peer discussions in order to lead to better patient care, all while remaining within the boundaries suggested by OIG?”
Mr. Dahl added, “COVID-19 and the vastly more virtual world brought on in response to the pandemic have accelerated the paradigm shift that has been taking place over the last several years as the DOJ and OIG have set their enforcement sights on speaker program activities. Even though the OIG stated in its Special Fraud Alert that it didn’t intend to discourage ‘meaningful’ education for HCPs by industry, it made abundantly clear that it views traditional speaker programs as inherently risky. Companies must closely evaluate both the legitimate business need for speaker programs and how they conduct their programs to effectively manage the risks identified by the OIG.”
I couldn’t agree more. Once COVID-19 curtailed speaker dinner programs, the trend became clear—virtual speaker programs are here to stay. I’m willing to bet that the OIG would agree that carefully selecting when to do speaker dinner programs and supplementing education with virtual speaker programs will be a win-win for the regulators, the HCPs, and marketers alike.
________________________________________________________
1 Office of Inspector General, Special Fraud Alert: Speaker Programs, November 16, 2020, https://oig.hhs.gov/fraud/docs/alertsandbulletins/2020/SpecialFraudAlertSpeakerPrograms.pdf
2 Acting Manhattan U.S. Attorney Announces $678 Million Settlement Of Fraud Lawsuit Against Novartis Pharmaceuticals For Operating Sham Speaker Programs Through Which It Paid Over $100 Million To Doctors To Unlawfully Induce Them To Prescribe Novartis Drugs; DOJ, July 1, 2020, https://www.justice.gov/usao-sdny/pr/acting-manhattan-us-attorney-announces-678-million-settlement-fraud-lawsuit-against
3 Federal Register, Vol. 68, No. 86, Monday, May 5, 2003, https://oig.hhs.gov/authorities/docs/03/050503FRCPGPharmac.pdf